If you want to know more information (such as product/process price, etc.), please contact us 24-hour telephone
This guide walks you through the full high purity quartz sand impurity removal process. It covers common impurities, how to pick the right methods, step-by-step processing, equipment tips, and a real-world case study ready for industrial use.

High purity quartz sand, with super high SiO2 levels, is a big deal for making glass, silicon chips, lenses, and top-notch semiconductors. To hit over 99.5% SiO2, you need a mix of physical and chemical tricks to kick out stuff like iron, titanium, aluminum, potassium, sodium, and clay. In places like Spruce Pine, North Carolina, miners chase this purity for tech giants, and every bit of junk you remove boosts the sand’s value.
Honestly, getting that ultra-clean sand feels like a treasure hunt—tough but worth it when you see the final product shine.
Iron (Fe): This sneaky metal tints the sand and messes with its electrical and optical qualities. Even a tiny 0.05% Fe2O3 can ruin a batch for high-end glass.
Titanium (Ti): It clouds clarity and causes flaws in silica glass, which is a no-go for fancy optics.
Aluminum & Feldspar (Al, K, Na): These raise the melting point and leave pesky lumps in the final product. Feldspar’s a common pain in Australian quartz deposits.
Clay & Organics: These sticky bits clog up flotation and acid steps. You’ve got to scrub them out early.

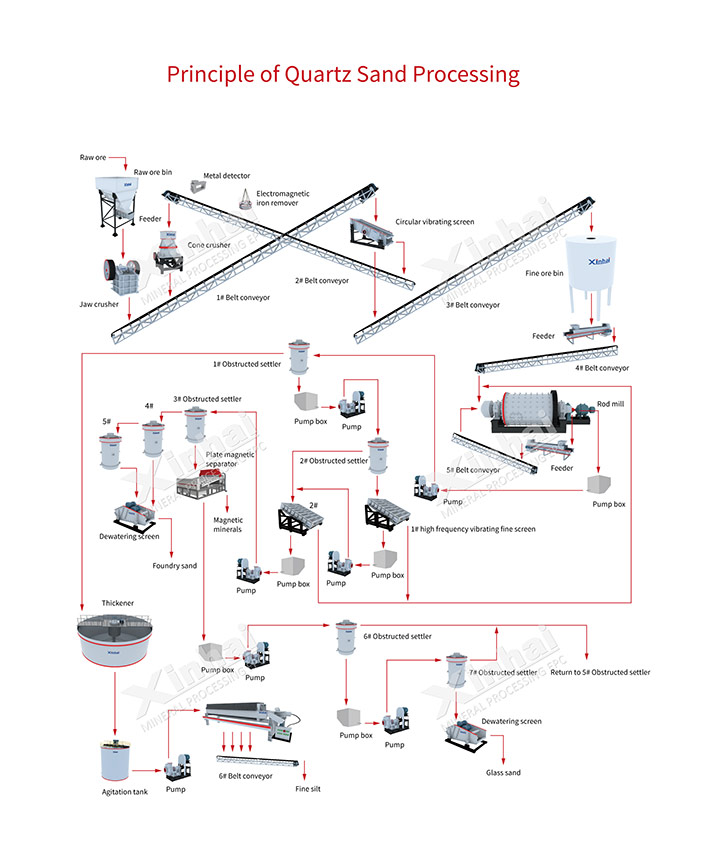
Here’s the basic path from raw sand to super clean, high purity quartz sand:
Screen and wash. This clears out big chunks and loose dirt.
Deslime and scrub hard. It knocks off clay and organic gunk.
Magnetic separation. This pulls out iron oxides like magnetite.
Flotation. It strips away feldspar, mica, and some titanium minerals.
Acid or alkali leaching. These dissolve leftover iron, titanium, and aluminum.
Polish with roasting or heat. This gets you to top-tier purity.
Dewater, dry, and sort. You end up with sand sized just right.
A plant in Norway follows this exact flow and hits 99.8% SiO2 from a 94% starting point. It’s a solid setup for most quartz deposits.
Magnetic separation is your first, budget-friendly move to yank out free iron oxides like magnetite or some hematite. For coarse or medium sand, a high-gradient magnetic separator or wet drum magnet does the job fast. It clears out magnetic junk and eases the load on later chemical steps. At a site in India, they cut iron content by 60% just with a wet drum before moving to leaching.
Attrition scrubbing is like giving the sand a rough bath. It shakes loose clay coatings and frees tiny impurities. Then, hydrocyclones or washing screens deslime the mix, pulling out fine muck. This step boosts flotation results and cuts acid use later. A Chinese operation saved 25% on acid by getting this right—small tweak, big payoff.

Flotation uses pH tweaks and special chemicals like soda ash to separate feldspar and mica from quartz. It also grabs some titanium-heavy bits. This cuts aluminum and potassium/sodium levels before leaching. In a South African plant, flotation dropped Al2O3 from 0.5% to 0.1% in one pass. You just need the right depressants to make it click.
Chemical leaching tackles stubborn iron, titanium, and aluminum locked in the sand. Here’s what works:
HCl or H2SO4 leaching: Great for iron. Keep temperature and acid strength balanced to avoid eating away silica. A German plant runs HCl at 60°C for two hours and drops Fe2O3 to 0.03%.
Alkali fusion or NaOH leaching: Good for aluminum and busting feldspar. Wash well after to clear residues.
Combined roast-leach: Roast with Na2CO3 (soda ash) to loosen impurities, then leach and rinse. This got a Brazilian site to 99.9% SiO2.
Quick tip: Watch temperature and timing close—too much silica loss jacks up costs and kills yield.
For super pure needs, roasting burns off leftover iron and turns tough impurities into forms you can wash out. Follow with a light acid rinse and lots of water. A Norwegian crew hit 99.95% SiO2 for semiconductors by roasting at 900°C before a final HCl dip.
| Stage | Equipment | Key parameters | Notes |
|---|---|---|---|
| Screening & washing | Vibrating screens, log washers | Feed<50 mm; wash water flow: optimized to avoid over-washing | Remove coarse impurities and salt/organic debris |
| Desliming | Hydrocyclones, classifiers | Cut size 45–75 µm depending on clay content | Reduces slimes to improve flotation & leach efficiency |
| Magnetic separation | Wet drum magnet, HGMS if needed | Field strength: 800–2000 Gauss (drum) / HGMS higher | First-line Fe removal—lowers acid demand downstream |
| Flotation | Mechanical flotation cells | pH control 8–11 (for feldspar depress), pulp density 25–35% solids | Use selective collectors/depressants for feldspar/mica |
| Chemical leaching | Agitated leach tanks, autoclaves (if needed) | Acid conc. 5–20% (HCl/H2SO4); T: 60–95°C; time: 1–4 h | Optimize to minimize silica loss |
| Dewatering & drying | Thickeners, filter press, rotary dryers | Final moisture<0.5–2% (depending on spec) | Product grading to meet end-use PSD |
| Method | Target impurities | Effectiveness | Pros | Cons |
|---|---|---|---|---|
| Magnetic separation | Magnetite, coarse Fe oxides | High for magnetic fraction | Low cost, simple operation | Ineffective for hematite or chemically bound Fe |
| Flotation | Feldspar, mica, some Ti phases | Medium–High | Selective removal of silicate gangue | Requires reagents; sensitive to slimes |
| Acid leaching / roast-leach | Residual Fe, Ti, Al | High (when optimized) | Deep purification to >99.5% SiO2 | Costly, requires acid handling and wastewater treatment |
| Attrition scrubbing | Clays & coatings | High for surface-bound impurities | Improves downstream results | Needs slurry handling and desliming |
Feed: 92.0% SiO2, Fe2O3 0.85%, TiO2 0.12%, Al2O3 0.45%.
Process applied: Wash first. Then scrub and deslime. Run drum magnetic separation. Float out feldspar. Leach with HCl at 60°C for two hours. Finish with thickening and filtration.
Result: Got 99.65% SiO2, Fe2O3 0.04%, TiO2 0.01%, Al2O3 0.06%. Kept 78% of the mass. A similar setup in Quebec hit 99.7% SiO2 with tighter controls, showing room to tweak.
Waste acid handling: Set up precipitation and neutralization loops. Recover metals if it pays—like a Canadian plant pulling $50,000 a year in copper from waste.
Water recycling: Closed loops cut water use and waste dumps. A South African site reused 85% of water, dodging tight local rules.
Worker safety: Acid needs strict rules—PPE, spill-proof tanks, and training. One slip in a US plant cost $20,000 in cleanup, so don’t skimp here.
First, try magnetic separation for loose iron oxides. Then hit it with acid leaching like HCl or H2SO4. For tough cases, roast and leach again to clear chemically stuck iron.
Yep, flotation works great. Use pH tweaks and the right chemicals to pull feldspar and mica away from quartz. A good setup can drop Al2O3 by 80%.
With physical and chemical steps, you can hit 99.5% to 99.9% SiO2. It depends on starting impurities and how fine you grind. Finer grinds push purity but cut yield.
It’s manageable with care. Treat wastewater, neutralize acids, and recover metals. Good design—like double-lined tanks—keeps spills and impacts low.
Need a tailored purification route?
For pilot testing, equipment picks, or a custom high purity quartz sand impurity removal process plan, contact us. We offer lab tests, pilot runs, and full EPC+M+O support.